PHANTOM PAINS AND PEMF'S
Table of Contents

SUMMARY
Pain following amputations (post-amputation pain – PAP) is very common and challenging to treat. There are many mechanisms that cause PAP. They involve brain, spinal and local aspects. The brain aspects involve reorganization of brain nerve patterns representing the amputated limb that result in phantom limb pain (PLP) and phantom sensations (PS). There can also be reorganization of spinal nerve patterns with the peripheral nerve injury associated with the amputation. In the stump area nerve damage causes inflammation, regenerative nerve sprouting, and increased nerve signals in the remaining limb or the development of a neuroma.
Treatment should be individualized. Treatments mostly include injection therapy, medications, alternative therapies, surgery, and prevention strategies. Treatments can be targeted to the brain, spinal cord or locally to the stump. Pulsed electromagnetic field (PEMF) therapy is a safe, low risk, noninvasive, externally applied therapy that has been found to be effective in helping PAP and PLP. This therapy may even be applied daily in the home setting for the best long-term results.
HISTORY
There is archaeological evidence for intentional amputations dating back at least 45,000 years. Major advancements in the care of amputations have happened as result of improvements in dealing with injuries from wars over the centuries. Weir Mitchell coined the term “phantom pain” when reporting on 86 Civil War amputees, where 90% had PLP. (Louis; Mitchell) PAP is considered one of the most challenging of all pain conditions to treat. After all, how can you treat a phantom? It is now recognized that the challenge stems from the fact that there are many physical mechanisms that lead to PLP.
HOW COMMON IS PAP?
In the US, in 2005 1.6 million people lost limbs. This is projected to increase to 3.6 million by 2050. (Hsu)
about 185,000 amputations are done every year. Vascular disease is the most common cause at 82%, followed by trauma [16%], cancer [0.9%] and congenital anomalies [0.8%]. At least 95% of those with limb loss experience PLP, PS or residual limb pain (RLP). Amputees have a hard time distinguishing between these different forms.
PLP is a painful or unpleasant sensation in the lost body part – the part that no longer exists. PLP can be felt as sharp, shooting or electrical lakes sensations or dull, squeezing, or cramping pain. It can be felt in the entire limb or just one area of the limb. PLP typically shows up within the first 6 months after amputation. By the time several years passes, about 85% can have PLP and can persist for years. PLP can change over time from a knife like/sticking sensation involving the entire missing limb to a burning/squeezing sensation in the further areas of the missing limb.
Published PLP rates range from 50% to 80% of limb amputees, with 5–10% of these individuals experiencing extreme pain (Batsford; Yin). Variations in prevalence are based on pre-amputation pain, amputation location, anesthetic and surgical procedure, sex, psychological factors, and time after amputation (Richardson; Aiyer). Almost immediately following the amputation of a limb, 90–98% of patients report experiencing a phantom sensation.
Nearly 75% of individuals experience the phantom as soon as anesthesia wears off, and the remaining 25% of patients experience phantoms within a few days or weeks. Literature indicates that majority of amputees suffer PLP in the year following their amputation, but with time declines from 72% at 8 days, 65% at 6 months and 59% at 2 years (Collins). Likewise, there are lower prevalence rates of phantom pain over time, 32% at 6 months, 26% at 1 year and a half, 23% at 2 years and a half and 27% at 3 years and a half after amputation (Bosmans).
RLP or “stump pain” is often felt as sharp, burning, electrical like or “skin-sensitive” pain in the incision, felt deep in the remaining limb or sometimes involve the whole remaining limb. Stump pain can happen about 74% of the time and like PLP can persist for years. The stump pain may be classified into multiple categories depending on cause. Stump pain is usually more bothersome right after amputation, with PLP becoming more dominant 1 – 12 months afterwards. There’s a big correlation between severity RLP and PLP.
PSs are nonpainful perceptions coming from the lost body part. They are common after the surgery. One third have sensations within 24 hours, three quarters within 4 days and 90% within 6 months. PSs can also happen after nerve injuries or spinal cord injuries without amputations. They can be sensed as movements of the amputated part that can be willed or spontaneous. There can be sensations related to the size, shape or position of the missing part, such as feeling that a foot is twisted.
There may also be sensations of touch, pressure, tingling, temperature, itching or vibration. They are more likely to involve sensations relating to the hands or feet. PSs can also occur from loss of other body parts, including about 25% of those with mastectomies. Another sensation is the sense of shortening of the amputated limb, so that it doesn’t feel the same in size or length. This is called telescoping and happens in one quarter to two-thirds of amputees.
PLP is further complicated by pre-existing orthopedic issues, such as low back pain from disk or spine problems, vascular insufficiency problems above the level of the body part being amputated, among others. PLP or stump pain may be related to a local neuroma in the stump or even to prosthesis-related pain. The latter happens especially over time as the stump changes shape and size and a prosthesis needs to be remolded or readjusted. Bone or graft infections are not uncommon as well.
MECHANISMS OF PLP
All the different parts of the body are represented by sensory nerves, grouped by area into “maps,” called homunuculi, that relate to the density of sensory neurons in a given body area. This image is one example of a cortical sensory map.
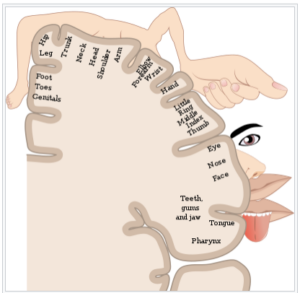
From: https://en.wikipedia.org/wiki/Cortical_homunculus
Brain related causes of PLP are due to reorganization of the nerve pathways in the somatosensory cortex of the brain surrounding the area that would normally represent the lost limb. For example, brushing the face of upper limb amputees can elicit PSs, because these areas are close to the area represented by the lost limb. Therefore, there would be spillover of sensory information into the area that still contains neurons representing the lost limb.
The neurons are still there but there is no sensory information coming to them from a lost limb. Brain imaging studies show that the area representing a lost hand is activated during movement of the remaining arm. In fact, stimulation of the brain in the brain area representing the lost limb can cause contraction of the rest of the extremity. The greater the size of the brain area of the lost sensation the more intense the PSs.
Sources of PLP from the stump can be from spontaneous nerve activity from the cut end of nerves. If this is the source of the PLP/stump pain, injection of local anesthetic will often give temporary relief. This spontaneous activity of the nerves in the stump can lead to the pain being made worse or initiated by emotional distress, exposure to cold, and other stimuli.
Spinal sources for PHP/PLP happen because of decreased nerve traffic into the spinal cord. With decreased nerve traffic into the spinal cord, the brainstem does not send down the normal inhibitory messages to decrease whatever sensory traffic remains. This situation can end up creating autonomous firing of spinal neurons, effectively becoming “sensory epileptic discharges” in the spinal cord. This is essentially “spinal reorganization”, comparable to cortical reorganization. Spinal reorganization is seen in lower limb amputees who develop new disc herniations or shingles infections.
There are many other spinal and brainstem changes that happen over time, including neuronal atrophy, that make the post amputation physical changes complex. As an example, even spinal anesthesia can rekindle previously inactive PLP.
CONVENTIONAL TREATMENTS
Treatments conventionally include spinal reorganization injections, medications, alternative therapies, surgery and attempts at prevention. Local injections, including anesthetics or botulinum, are more useful in RLP or stump pain and often give little sustainable help for PLP.
Medications are variably helpful, may create addiction, immune challenges and other side effects. They rarely cure the problem, which can recur when the medication is stopped. Because PAP treatments are so challenging and have limited benefits, alternative therapies have been used frequently. Some include psychological therapy, cognitive behavioral therapy, hypnosis, etc., there is little evidence for long-term benefit. Mirror therapy has gained popularity and can have short-term benefits with improved pain scores. However, there is no evidence for long-term benefits.
Surgical therapies, especially peripheral nerve reconstruction, have mixed results, can have significant complications and side effects and should only be considered as a last resort, with the failure of other less invasive approaches, including neuromodulation.
Prevention approaches have been used preoperatively, during surgery or within 2 weeks of the amputation. Some of these approaches have included epidural injections, regional nerve blocks, infusions and TENS. Long-term benefits have been limited, and one review found that there was limited evidence of benefit for treatments in the acute postoperative period. Results have been inconsistent and variable, especially for longer-term benefits.
Amputation not only results in the loss of neural input/feedback from the amputated limb but also produces a loss of visual and tactile information related to the limb. The brain influences that normally inhibit pain may be further reduced by the absence of information from these external sources that might otherwise confirm or reduce the perception of pain arising from the periphery (for example, a phantom limb in a painful position or a “crawling” sensation on the skin). Thus, some forms of PLP may arise, in part, from a release from inhibitory control (that is, disinhibition).
Mirror therapy (MT) is a long-standing treatment for PLP and is to work by restoring normal somatosensory and visual inputs to the brain. (Aternali) Individuals were instructed to move both the intact and phantom limb at the same time during MT while viewing the reflected image of the intact limb in the mirror in the place of where the amputated limb would have been.
A recent neuroimaging study of lower limb amputees with PLP found that viewing mirror images of feet (but not hands) in the foot area of sensorimotor cortex of the brain opposite to the amputated limb as well as in posterior parietal cortex produced better results. Unfortunately, the intensity of the PLP and the increased visual responsiveness no longer worked after 4 weeks of MT. Seeing the limb (via MT) re-establishes the normal inhibitory sensation control processes.
MT restores inhibitory control over brain cells receiving sensory information in these and other brain regions and reduces abnormal brain activity, which contribute to the PLP, and thereby reduces pain. The results of the most recent studies evaluating the efficacy of MT for PLP are not promising, especially long-term. Overall, MT does not appear to reduce PLP to a greater degree than control or other known treatments. At least, it’s inexpensive.
PEMF RESEARCH
PEMF treatment can be applied to the stump, the spine or the brain. For the treatment of the PLP itself, there is almost no research using lower intensity PEMF systems. There is significant evidence for the value of PEMFs in dealing with chronic pain in general, whether treating the brain or periphery. (Pawluk, 2015) PEMFs are also established for helping wound healing (Pawluk, 2017)
There is some research on the value of transcranial magnetic stimulation (TMS) for PLP.
One group (Nardone) did a systematic review using PEMFs in individuals with PLP and PS. Brain imaging found an enlarged increased cortical excitability in the opposite hemisphere to the PLP. However, these cortical excitability changes and PLP do not correlate with each other. TMS influences brain function when used repetitively. TMS to the opposite brain area led to a transient reduction in pain intensity. Stimulation can be to the opposite motor cortex. The natural, endogenous painkilling chemical, Beta-endorphin, increases significantly after stimulation to the opposite motor cortex.
Another clinical review (García-Pallero) also found that TMS was effective in decreasing pain in PLP and symptoms of anxiety and depression compared to simulated TMS or placebo. Pain syndromes other than PLP can be improved by TMS. Stimulation of the opposite parietal cortex decreased pain intensity of up to 10 minutes, with no clear effect on pain in other cortical areas. Even stimuli used for 3 consecutive weeks on the opposite parietal cortex did not result in lasting improvements. PLP in the parietal cortex is due to dysfunctional activity. In 27 patients with PLP after unilateral amputation, 5 consecutive days of 10 minutes of real TMS over the hand region of motor cortex every day versus sham stimulation, VAS (Visual Analogue Scale) and LANSS scores (Leeds Assessment of Neuropathic Symptoms and Signs) decreased significantly.
Depression and anxiety levels decreased significantly. Finally, beta-endorphin was assessed 1 to 2 hours after five sessions and significantly increased after real stimulation. In TMS treatment of PLP in landmine victims, of 49 individuals, 26 of whom received real TMS over the opposite side brain motor strip (M1), there was no significant overall average visual analog pain scale (VAS) differences at 15 or 30 days. However, a decrease in VAS was seen by more than 30% of the individuals at both 15 days and 30 days.
Another clinical trial found that TMS caused significantly better pain relief 15 days after active treatment versus placebo stimulation. But this benefit was gone 30 days after treatment. 70% of those in the active group had clinically significant pain relief (> 30% relief), compared to 41% in the sham group. In this study, anxiety and depression symptoms weren’t different. This trial again points out the need for longer-term PEMF treatment.
As stated earlier, PAP or PLP may be affected by spinal cord functional signal traffic disinhibition, even without spinal cord injury, suggesting a possible benefit from spinal cord treatment. PEMFs have been found to have significant regenerative effects on spinal cord injuries and function. (Ross; Kumar) PEMFs can indirectly electrically activate spinal circuits with the noninvasive pulsed electromagnetic field (PEMF) applied over intact vertebrae, safely passing through the tissues to reach the spinal cord. So, when direct brain stimulation or direct stump stimulation doesn’t work to reduce PAP or PLP, spinal cord stimulation can be attempted or added. This should also be considered when the benefits from direct brain stimulation or stump stimulation are less than optimal.
PEMF TREATMENT RECCOMENDATIONS
Phase 1. Moderate to high intensity PEMF therapy to the stump. The researched therapy with the best noninvasive therapy results for PLP is high intensity transcranial brain stimulation. Since stump pain is the easiest to access and a common cause of not only local pain but also phantom limb pain, treatment of the stump as an initial strategy may be simplest and easiest. For this purpose, moderate to high intensity PEMF therapy daily in the home setting may be best strategy, especially if started as soon after amputation as possible. In this case local PEMF therapy may prevent the onset of phantom limb pain, which often does not start for some time after the amputation.
If this resolves the local pain and/or phantom limb pain, exploring other approaches may not be necessary. As commonly happens, early results can be short-lived, especially if therapy is not continued. In this case it may be best to do local therapy for several months, even after the pain has been resolved, so that imperceptible, deep tissue healing, especially of nerves, can still produce results. A local, portable PEMF device may work well to a stump and can be used for hours at a time daily. Higher intensity PEMF devices can be combined with lower intensity portable equipment for better and faster results.
Phase 2. High intensity transcranial brain stimulation. While local pain problems may be resolved naturally or with physical therapy, after amputation, phantom limb pain may still begin at any time without any contribution from the stump. If that happens, the next phase of therapy should be transcranial stimulation. For this purpose, high intensity PEMF, done daily for a minimum of 30 minutes twice a day, whether it’s to the opposite side of the brain to the amputation or the same side, doesn’t matter and will be a matter of trial and error for which works best.
While not researched, it may be possible to alternate sides of the brain on the same day of treatment. Treatment should be continued until the pain is resolved. If treatment is stopped and the pain returns, then treatment should be started again. It is hoped that, if this happens, re-starting treatment may produce results faster. Phantom limb pain or phantom sensations may continue indefinitely, hopefully lessened by PEMF therapy, which is the rationale for owning a home-based PEMF therapy system. Getting the assistance of a PEMF expert may be best to achieve the best results.
Phase 3. High intensity spinal stimulation. If treatment is not either fully or partially successful with local and/or transcranial PEMF treatment, treating the spine may be considered next. This may be done at the back of the neck, mid back or the lower back. Trial and error may give the best indication for which treatment area produces the best results. As with the other therapies, treatment may need to be at least twice a day for months before a conclusion can be reached about the effectiveness. As with other therapies, initial results may not continue, so treatment may have to be reinitiated again for some interval. This may happen multiple times.
Phase 4. High intensity stump, brain (transcranial) and spinal stimulation.
It’s possible that after all the above approaches have been tried individually with less-than-optimal results, it may be necessary to treat 2 or 3 different areas (local, brain and/or spinal cord) either at the same treatment session or during the same day. This will need to be a matter of trial and error.
GOING LOW AND SLOW
Because PEMF therapy stimulates tissues to heal, initial reactions, such as increased pain, are common and resolve with continued treatment as healing of the tissue, reduction in nerve irritability and cortical excitability are facilitated. Nevertheless, it may be necessary to start off with lower intensities and shorter treatment times, which then can be gradually increased as symptoms improve, and healing occurs. Examples of going low and slow are available in the blog section on DrPawluk.com.
ASSISTANCE WITH PEMF THERAPY
Assistance with PEMF therapy, whether for purchase of a device or ongoing therapy support, can be obtained through DrPawluk.com. Another good resource for general support for PEMF therapy can be obtained through the book Supercharge Your Health with PEMF therapy.
CONCLUSION
Persistent pain following amputation is very common. It is also very frustrating to treat with limited benefits and minimal risk. There are 3 types of persistent pain, stump pain, phantom limb pain and pain initiated or maintained by spinal cord processes. Phantom limb pain is most commonly and most effectively managed by neuromodulation of the brain, using pulsed electromagnetic field therapy. PEMFs can be obtained and used in each of the common settings of post amputation pain. There will need to be some significant trial and error to determine the best protocols for any given individual. It is very likely that treatment will need to be continued for extended periods of time, even perhaps indefinitely.
REFERENCES
- Aternali A, Katz J. Recent advances in understanding and managing phantom limb pain. F1000Res. 2019 Jul 23;8:F1000 Faculty Rev-1167.
- Hsu E and Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121-36.
- Garcia-Pallero MÁ, Cardona D, Rueda-Ruzafa L, et al. Central nervous system stimulation therapies in phantom limb pain: a systematic review of clinical trials. Neural Regen Res. 2022 Jan;17(1):59-64.
- Kumar S, Dey S, Jain S. Extremely low-frequency electromagnetic fields: A possible non-invasive therapeutic tool for spinal cord injury rehabilitation. Electromagn Biol Med. 2017;36(1):88-101.
- Louis ED, York GK. Weir Mitchell’s observations on sensory localization and their influence on Jacksonian neurology. Neurology. 2006 Apr 25;66(8):1241-4.
- Mitchell SW, Morehouse GR, Keen WW. Gunshot Wounds and other Injuries of Nerves. Philadelphia: JB Lippincott and Co; 1864.
- Nardone R, Versace V, Sebastianelli L, et al. Transcranial magnetic stimulation in subjects with phantom pain and non-painful phantom sensations: A systematic review. Brain Res Bull. 2019 May;148:1-9.
- Pawluk W and Layne CJ. Power Tools for Health: how magnetic fields (PEMFs) help you. Publ. Friesen Press, 2017.
- Pawluk W. Magnetic fields for pain control, chapter 17. In Electromagnetic Fields in Biology and Medicine, CRC Press, publication Jan 2015, Markov M, Editor.
- Ramachandran VS and Hirstein W. (1998). “The perception of phantom limbs. The D. O. Hebb lecture”. Brain : A Journal of Neurology. 121 (9): 1603–1630.
- Ross CL, Syed I, Smith TL, Harrison BS. The regenerative effects of electromagnetic field on spinal cord injury. Electromagn Biol Med. 2017;36(1):74-87.
- Louis ED, York GK. Weir Mitchell’s observations on sensory localization and their influence on Jacksonian neurology. Neurology. 2006 Apr 25;66(8):1241-4.